Tomasz ZÄ…bkowski
Military Medical Institute
Poland
Title: Evaluation of the clinical indications, adverse drug reactions, and finasteride use in patients with benign prostatic hyperplasia in Poland
Biography
Biography: Tomasz ZÄ…bkowski
Abstract
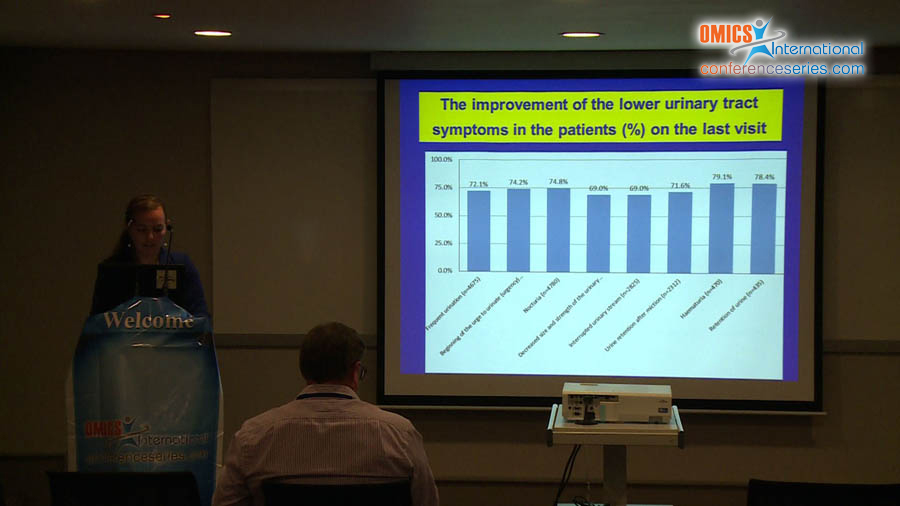
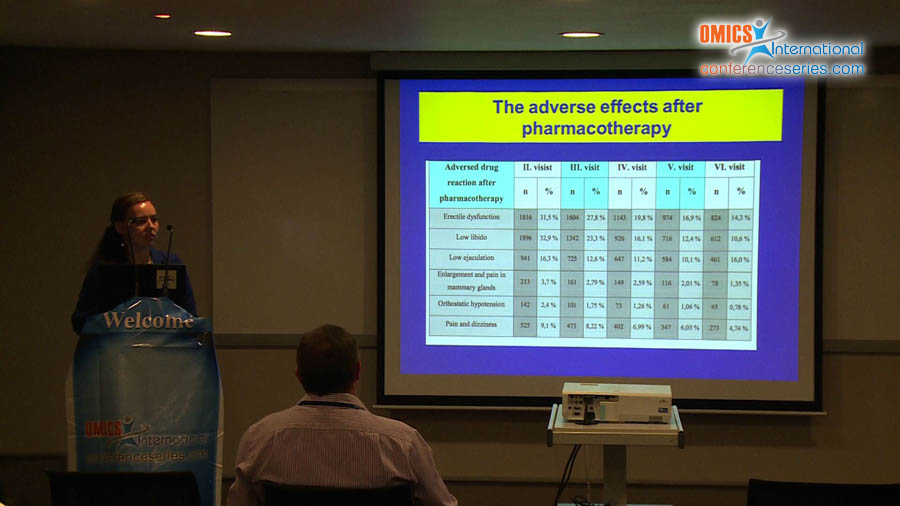
Benign prostatic hyperplasia (LUTS/BPH) is one of the most common urinary disorders in elderly men. Over the recent years, the number of surgical interventions performed in pharmacotherapy has significantly reduced because of the increased efficacy of conservative therapy, including combination treatment mostly with 2 groups of drugs, namely, alpha-1-adrenolitics and other 5-alpha-reductase blockers, with a different pharmacological activity. The aim of this study was to evaluate the clinical indications, adverse drug reactions, and finasteride use in patients with diagnosed BPH in Poland. We conducted a clinical trial from November 2009 to November 2010 that included 5751 patients who were enrolled in 46 urological centers in Poland. The researchers who conducted the clinical trial were urologists from different regions of Poland. The clinical trial involved 6 follow-up visits. The mean age of the patients was 67 years (range, 45–93 years; median, 67.00; SD, 8.507). The inclusion criteria were as follows: LUTSs, finasteride therapy for at least 2 weeks, age >40 years, and BPH. Patients self-reported data on LUTSs, the symptom frequency, concurrent diseases, and intensification of urinary system symptoms. In addition, additional examinations were performed, including prostate-specific antigen test, urinary tract ultrasonography with evaluation of residual urine and prostate, and uroflowmetry. The study did not exclude data on the combined treatment with finasteride and alpha-1-adrenolitics. Finasteride was demonstrated to be effective, as evidenced by the significant decrease in TPV by 40% even after 12 months, and improvement in maximum flow rate, decrease in nocturia, and improvement in QoL.