Giovanni Furlan
Helsinn Birex Pharmaceuticals
Ireland
Title: Inefficiencies in pharmacovigilance: What we can do now and what could be done in the future
Biography
Biography: Giovanni Furlan
Abstract
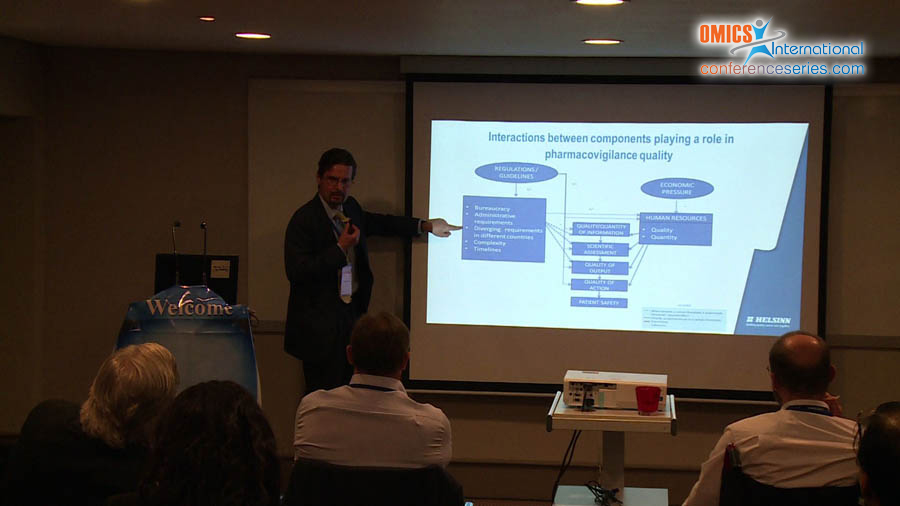
In the western world, at government level it is being recognized that the regulations to develop and licence a new drug are probably too demanding. In fact, the pharmaceutical industry is the most regulated and drug safety is probably the most regulated area within this industry. This not only slows down the time for developing and registering a new drug, but also increases the costs for maintaining a drug registration. In a resource constrained world, the question of whether the requirements to prepare multiple, partially overlapping, documents is in the interest of patient safety or whether it represents a risk to patient safety is not a trivial one. EU regulations require the preparation of signal detection reports, Periodic Safety Update Reports, Developmental Safety Update Reports, Risk Management Plans, addendums to the clinical overview, Investigators’ Brochures and, with the new clinical trial regulation, benefit-risk information will need to be included in the protocol and in the Investigational Medicinal Product Dossier. Furthermore, the reporting requirements diverge between ICH countries and are even more so if non-ICH countries are considered. The preparation of so many documents drains resources from the scientific evaluation of safety information simply to fulfil bureaucratic requirements. Therefore, there is a need to further harmonize pharmacovigilance requirements and to unify the many overlapping documents in one single modular drug safety master file. Furthermore, pharmaceutical companies also can improve their efficiency have to improve their efficiency: a low hanging fruit in this regard is the unification of the drug safety and clinical databases, which would have multiple benefits, such as the elimination duplicate data processing, the need for reconciliation, the expenses of validating and managing two separate databases and the duplication of queries to investigators.
Speaker Presentations
Speaker PDFs
Speaker PPTs Click Here