Theme: Global Pharmacovigilance Approaches and Innovations for Patient Benefit Risk Management
Pharmacovigilance 2018
We solicit your gracious presence at the upcoming 11th International Conference and Exhibition on Pharmacovigilance and Drug Safety scheduled to happen during June 21-22, 2018 in London, UK focussing on the advancements in emerging research and technologies in pharmacovigilance, clinical trials and risk management.
The field of Pharmacovigilance is growing rapidly and its development is making tremendous impacts in medical sciences and pharmaceuticals. Pharmacovigilance 2018 emphasizes on how the importance and significance can be gauged by the fact that it has made huge advancements over the course of time and is continuing to influence various sectors.
Theme: “Global Pharmacovigilance Approaches and Innovations for Patient Benefit Risk Management”
Track 1: Pre-Clinical and Clinical Trials
Clinical trials allow the drug to be tested for safety by different ethnic population. Due to the higher medical needs and increasing disease prevalence, developing countries are becoming a hub for clinical trial execution. The clinical trials market has been estimated to reach USD 14.2 billion in 2016 and is projected to reach around USD 22 billion by the year 2021, and the annual growth rate of (7.5%). There are additionally numerous clinical trials started by scholarly clinical scientists. Whether started by industry or by scholastic clinical examiners and research is frequently performed in national, European and overall consortia, which can be expansive ones. Clinical research brings up moral and security issues. Clinical research is exceedingly controlled. To encourage and coordinated efforts crosswise over fringes.
Related Pharmacovigilance Congress | Pharmacovigilance Events
9th Conference on Pharmacology during September 04-06, 2017 at Paris, France; 3rd Conference on Advanced Clinical Research September 20-21, 2017 Dublin, Ireland; 9th Pharmaceutical Chemical Analysis Conference October 02-03, 2017 Vienna, Austria; 5th Conference on Pain Research and Management October 05-06, 2017 London, UK; 20th Conference on Pharmaceutical Biotechnology December 07-09, 2017 Madrid, Spain; 12th Conference on Pharmaceutical Sciences February 26- 27, 2018 London, UK; 16th Conference on Pharmaceutics March 19-21, 2018 Berlin, Germany; 11th Biosimilars Conference April 26-27, 2018 Rome, Italy; 10th Pharma Conference May 07-09, 2018 Frankfurt, Germany; 4th Conference on Marine Drugs June 11-13, 2018 Rome, Italy
Related Associations or Societies:
Society for Clinical Trials (SCT), Association of Clinical Research, The Society of Clinical Research Associates, Association of Clinical Research Organization, Clinical Research Society, Indian Society for Clinical Organization Research (ISCR)
Track 2: Adverse Drug Reactions
Adverse drug reactions can be considered a form of toxicity or enhanced drug effects that occur during appropriate use (eg, when drug metabolism is temporarily inhibited by a disorder or another drug). In the US, 3 to 7% of all hospitalizations are due to adverse drug reactions. ADRs occur during 10 to 20% of hospitalizations; about 10 to 20% of these ADRs are severe. Incidence of death due to ADRs is unknown; suggested rates of 0.5 to 0.9% may be falsely high because many of the patients included had serious and complex disorders. Incidence and severity of adverse drug reactions vary by patient characteristics (eg, age, sex, ethnicity, coexisting disorders, genetic or geographic factors) and by drug factors (eg, type of drug, administration route, treatment duration, dosage, and bioavailability). Incidence is higher with advanced age and polypharmacy.
Related Pharmacovigilance Conferences | Pharmacovigilance Meetings
9th Conference on Pharmacology September 04-06, 2017 Paris, France; 3rd Clinical Trials Conference September 20-21, 2017 Dublin, Ireland; Pharmacovigilance Conferences 2018 Asia, 9th Conference on Pharmaceutical Chemical Analysis October 02-03, 2017 Vienna, Austria; 5th Conference on Pain Research and Management October 05-06, 2017 London, UK; Pharmacovigilance Conferences 2018, Conference on Pharmaceutical Biotechnology December 07-09, 2017 Madrid, Spain; 12th Conference on Pharmaceutical Sciences in Pharma Industry February 26-27, 2018 London, UK; 16th Conference on Drug Delivery Systems March 19-21, 2018 Berlin, Germany; 11th European Biosimilars Conference April 26-27, 2018 Rome, Italy; Pharmacovigilance Conferences 2018 Asia,10th Pharma Conference May 07-09, 2018 at Frankfurt, Germany; 4th Conference on Natural Products June 11-13, 2018 Rome, Italy
Related Associations or Societies:
American Association of Pharmaceutical Scientists, Indian Pharmaceutical Association, Association of Clinical Research, The International Society of Pharmacovigilance, Canadian Society of Pharmaceutical Sciences (CSPS), Association of Clinical Research Organization (ACRO)
Track 3: Pharmacovigilance and Risk Management
Pharmacovigilance and Risk Management comprises set of pharmacovigilance activities and interventions designed to identify, characterise, prevent or minimise risks relating to medicinal and therapeutic products including the assessment of the effectiveness of their clinical interventions and combination therapies. Drug industry need to promote companies in pharmacovigilance practice to use information technology and to review softwares used in pharmacovigilance and clinical trials. Monitoring unlicensed, off labels and orphan drugs is major task in risk management. Many experts from different pharmacovigilance CRO's , pharmacovigilance service providers and industrial delegates are participating in this conference to share their knowledge and discuss about the new updates.
Related Pharmacovigilance Conferences | Pharmacovigilance Meetings
9th Pharmacology Conference September 04-06, 2017 Paris, France; 3rd Conference on Clinical Research September 20-21, 2017 Dublin, Ireland; Pharmacovigilance Conferences 2018 Asia, 9th Pharmaceutical Analysis Conference October 02-03, 2017 at Vienna, Austria; 5th Conference on Pain Research October 05-06, 2017 London, UK; Pharmaceutical Biotechnology Conference December 07-09, 2017 Madrid, Spain; Pharmaceutical Sciences Conference February 26- 27, 2018 London, UK; Pharmacovigilance Conferences 2018, 16th Novel Drug Delivery Systems Conference March 19-21, 2018 Berlin, Germany; Pharmacovigilance Conferences 2018 USA, 11th Biosimilars Conference April 26-27, 2018 Rome, Italy; 10th Pharma Conference May 07-09, 2018 Frankfurt, Germany; 4th Conference on Marine Drugs June 11-13, 2018 Rome, Italy
Related Associations or Societies:
The International Society of Pharmacovigilance (ISOP), Pharmaceutical Information and Pharmacovigilance Association (PIPA), Association of Clinical Research Organization (ACRO), Indian Pharmaceutical Association, Association of Clinical Research
Track 4: Good Pharmacovigilance Practice
The role of Good Pharmacovigilance Practice and Pharmacoepidemiology in Risk Management is mainly to increase the probability of beneficial effects of a drug in a population than the probability of adverse effects and to maintain the Good Reporting Practices by avoiding the major problems in risk management. Also it is important to concentrate on Signal investigation by gathering the information on new or unknown drug effects that is potentially caused by a medicine and that finally should lead to ensuring safety. The pharmacovigilance and clinical trials services providing companies should have the Pharmacovigilance certification.
Related Pharmacovigilance Conferences| Pharmacovigilance Meetings
9th Pharmacology Conference September 04-06, 2017 Paris, France; 3rd Conference on Clinical trials September 20-21, 2017 Dublin, Ireland; Pharmacovigilance Conferences 2018, 9th Pharmaceutical Chemical Analysis Conference October 02-03, 2017 Vienna, Austria; 5th Pain Research Conference October 05-06, 2017 London, Pharmacovigilance Conferences 2018 USA, UK; Pharmaceutical Biotechnology Conference December 07-09, 2017 Madrid, Spain; Pharmaceutical Sciences in Pharma Industry Conference February 26- 27, 2018 London, UK; Pharmaceutics and Drug Delivery Systems Conference March 19-21, 2018 Berlin, Germany; 11th Biosimilars Conference April 26-27, 2018 Rome, Italy; 10th European Pharma Conference May 07-09, 2018 Frankfurt, Germany; Pharmacovigilance Conferences 2018 Asia, 4th Conference on Natural Products June 11-13, 2018 Rome, Italy
Related Associations or Societies:
American Association of Pharmaceutical Scientists, Indian Pharmaceutical Association, Association of Clinical Research, The International Society of Pharmacovigilance, Canadian Society of Pharmaceutical Sciences (CSPS), Association of Clinical Research Organization (ACRO)
Track 5: Pharmacy Practices and its Challenges
Pharmacy practice is the field of pharmacy which involves developing the professional roles of pharmacists. It includes Disease-state management, Clinical drug interventions, Pharmacy professional development and pharmaceutical care, pharmaceutical compounding and health psychology, patient care, drug abuse prevention, prevention of drug interactions or minimisation of adverse events and drug incompatibility and community pharmacy.
Related Pharmacovigilance Conferences| Pharmacovigilance Meetings
5th Pharmaceutical Sciences Conference; 8th Conference on Pharmacology, August 07-09, 2017, Pharmacovigilance Conferences 2018, Paris, France; 3rd Conference on Clinical Research September 20-21 Dublin, Ireland; Pharmacovigilance Conferences 2018 USA, 8th Pharmaceutical Analysis Conference September 25-27, 2017 at Vienna, Austria
Related Associations or Societies:
The Society of Clinical Research Associates (SOCRA), Clinical Research Society, Society for Clinical Trials (SCT), Associates of Clinical Research, Association of British Pharmaceutical Industry, Canadian Society of Pharmaceutical Sciences (CSPS)
Track 6: Biopharmaceutical Sciences
In Clinical Pharmacology and Biopharmaceutics track we will discuss about the Rational drug management of cancer, diabetes and cardiovascular disorders, and Management of psychiatric disorders and autoimmune disorders. Along with clinical trials Bioavailability and bioequivalence studies also plays major role in clinical research.The global market for Biopharmaceutics in 2013 was $305.1 million, which is expected to reach about $326.3 million by year-end 2014. The projected PAT instrumentation market is expected to be valued at around $450.6 million by 2019 at a compound annual growth rate (CAGR) of 6.7% for the period of 2014 to 2019.
Related Pharmacovigilance Conferences| Pharmacovigilance Meetings
9th Pharmacology Conference September 04-06, 2017 Paris, France; 3rd Clinical trials Conference September 20-21, 2017 Dublin, Ireland; Pharmacovigilance Conferences 2018 Asia, 9th Chemical Analysis Conference October 02-03, 2017 Vienna, Austria; 5th Conference on Pain Research October 05-06, 2017 London, UK; 20th Conference on Biotechnology December 07-09, 2017 Madrid, Spain; Pharma Industry Conference February 26- 27, 2018 London, UK; 16th Drug Delivery Conference March 19-21, 2018 Berlin, Germany; 11th Biosimilars Conference April 26-27, 2018 Rome, Italy; Pharmacovigilance Conferences 2018 USA, 10th Conference on Pharma May 07-09, 2018 Frankfurt, Germany; 4th Conference on Drugs and Natural Products June 11-13, 2018 Rome, Italy
Related Associations or Societies:
Association of British Pharmaceutical Industry, Canadian Society of Pharmaceutical Sciences (CSPS), Pharmaceutical Information and Pharmacovigilance Association (PIPA), American Association of Pharmaceutical; Scientists, American Society of Pharmacognosy
Track 7: Clinical Trials on Various Disorders
Clinical Trials on various diseases include Clinical Trials in long chronic diseases like AIDS, Benign and Malignant Tumours, Cardiovascular diseases, Diabetes, Clinical Trials on Monoclonal and Polyclonal Antibodies, Neurological, Physiological and Psychological Disorders
Related Pharmacovigilance Congress | Pharmacovigilance Events
9th Pharmacology Conference September 04-06, 2017 Paris, France; Pharmacovigilance Conferences 2018, 3rd Clinical trials Conference September 20-21, 2017 Dublin, Ireland; 9th Pharmaceutical Chemical Analysis Conference October 02-03, 2017 Vienna, Austria; 5th Conference on Pain Research and Management October 05-06, 2017 London, UK; Pharmacovigilance Conferences 2018 USA, 20th Pharmaceutical Biotechnology Conference December 07-09, 2017 Madrid, Spain; 12th Conference on Sciences February 26- 27, 2018 London, UK; 16th Pharmaceutics and Novel Drug Delivery Systems Conference March 19-21, 2018 Berlin, Germany; 11th Biosimilars Conference April 26-27, 2018 Rome, Italy; Pharmacovigilance Conferences 2018 Asia, 10th Pharma Conference May 07-09, 2018 Frankfurt, Germany; 4th Natural Products Conference June 11-13, 2018 Rome, Italy
Related Associations or Societies:
The Society of Clinical Research Associates (SOCRA), Clinical Research Society, Society for Clinical Trials (SCT), Associates of Clinical Research, Association of British Pharmaceutical Industry, Canadian Society of Pharmaceutical Sciences (CSPS)
Track 8: Data Quality Management and Analysis
Good data quality management in pharmacovigilance can be relied only on information gathered from the collection of individual case safety reports and other pharmacoepidemiological data. Quality management consists of quality planning, quality control, quality assurance and quality improvements. The pharmacovigilance data processing cycle starts with data collection and, in computerised systems, data entry; the next step is data storage and maintenance; followed by data selection, retrieval and manipulation. The resulting data output is analysed and assessed. Finally, conclusions are drawn and decisions are made. The quality of a pharmacovigilance data system can be defined as a measure of excellence or a state of being free from defects, deficiencies and significant variations and the data quality management includes all the activities that organizations use to direct, control and coordinate the quality of data.
Related Pharmacovigilance Congress | Pharmacovigilance Events
9th Conference on Pharmacology September 04-06, 2017 Paris, France; Pharmacovigilance Conferences 2018, 3rd Clinical Research Conference September 20-21, 2017 Dublin, Ireland; 9th Pharmaceutical Chemical Analysis Conference October 02-03, 2017 Vienna, Austria; 5th Conference on Pain Research October 05-06, 2017 London, UK; Pharmacovigilance Conferences 2018 USA, 20th Conference on Pharmaceutical Biotechnology December 07-09, 2017 Madrid, Spain; 12th Congress on Pharmaceutical Sciences and in Pharma Industry Conference February 26- 27, 2018 London, UK; 16th Drug Delivery Systems Conference March 19-21, 2018 Berlin, Germany; 11th Biosimilars Conference April 26-27, 2018 Rome, Italy; 10th Pharma Conference May 07-09, 2018 Frankfurt, Germany; Pharmacovigilance Conferences 2018 Asia, 4th Conference on Marine Drugs June 11-13, 2018 Rome, Italy
Related Associations or Societies:
Pharmaceutical Information and Pharmacovgilance Association (PIPA), American Association of Pharmaceutical; Scientists, American Society of Pharmacognosy , Association of Clinical Research Organization (ACRO), Society for Clinical Trials (SCT), Association of Clinical Research
Track 9: Pharmacovigilance Significance & Scope
Concept of Pharmacovigilance and its Significance enhances the impact of pharmacovigilance on patient welfare and public health and to know what is pharmacovigilance. This track gives a brief discussion on Pharmacovigilance role in healthcare system. Pharmacovigilance legislation gives an outlook on the rules and laws to follow in Pharmacovigilance practice. The Role of pharma industries in the improvement of pharmacovigilance system is very crucial to maintain the safety data, Detection and Evaluation of drug safety signals through manual and medical devices reporting. Pharmacovigilance scope also deals as Ecopharmacovigilance (EPV), pharmacoenvironmentology and pharmacovigilance in herbal medicines.
Related Pharmacovigilance Conferences| Pharmacovigilance Meetings
9th Pharmacology Conference September 04-06, 2017 Paris, France; Pharmacovigilance Conferences 2018 Asia, 3rd Advanced Clinical Research Conference September 20-21, 2017 Dublin, Ireland; 9th Pharmaceutical Analysis Conference October 02-03, 2017 Vienna, Austria; 5th Pain Research Conference October 05-06, 2017 London, UK; Pharmacovigilance Conferences 2018 USA, 20th Pharmaceutical Biotechnology Conference December 07-09, 2017 Madrid, Spain; 12th Conference on Pharma Industry February 26- 27, 2018 London, UK; 16th Conference on Novel Drug Delivery March 19-21, 2018 Berlin, Germany; 11th Biosimilars Conference April 26-27, 2018 Rome, Italy; 10th Pharma Conference May 07-09, 2018 Frankfurt, Germany; Pharmacovigilance Conferences 2018, 4th Conference on Marine Drugs June 11-13, 2018 Rome, Italy
Related Associations or Societies:
American Association of Pharmaceutical Scientists, Indian Pharmaceutical Association, Association of Clinical Research, The International Society of Pharmacovigilance, Canadian Society of Pharmaceutical Sciences (CSPS), Association of Clinical Research Organization (ACRO)
Track 10: Diversity in Industrial Clinical Trials and Clinical Research
The clinical trial industry is constantly evolving with diversified clinical research technologies and new clinical studies are being launched at an ever-growing pace. Clinical trials have always been a vital part of the medicine development process, as they provide clinical data on the best ways for treating pathological disorders and diseases. The importance of diversity in industrial clinical trials and clinical research is to ensure that industrial clinical trials are doing due diligence and being as strategic as possible in their results. This diversity makes us to better understand the unmet medical needs of patients.
Related Pharmacovigilance Congress | Pharmacovigilance Events
9th Conference on Pharmacology September 04-06, 2017 Paris, France; Pharmacovigilance Conferences 2018, 3rd Conference on Clinical Research September 20-21, 2017 Dublin, Ireland; 9th Pharmaceutical Analysis Conference October 02-03, 2017 Vienna, Austria; 5th Conference on Pain Research October 05-06, 2017 London, UK; Conference on Biotechnology December 07-09, 2017 Madrid, Spain; 12th Pharmaceutical Sciences Conference February 26- 27, 2018 London, UK; 16th Conference on Pharmaceutics March 19-21, 2018 Berlin, Germany; Pharmacovigilance Conferences 2018 USA, 11th Biosimilars Conference April 26-27, 2018 Rome, Italy; 10th Pharma Conference May 07-09, 2018 Frankfurt, Germany; Pharmacovigilance Conferences 2018 Asia, 4th Conference on Marine Drugs June 11-13, 2018 Rome, Italy
Related Associations or Societies:
Society for Clinical Trials (SCT), Association of Clinical Research, The Society of Clinical Research Associates, Association of Clinical Research Organization, Clinical Research Society, Indian Society for Clinical Organization Research (ISCR)
Track 11: Clinical Research and Statistics
In Clinical Research, Statistics plays a prominent role in regulatory submissions. Statistical analysis of Pharmacovigilance can be achieved by several guidelines mainly ICH guidelines. Adverse Drug Reactions reports can also be considered for the regulatory submission.
Related Pharmacovigilance Congress | Pharmacovigilance Events
9th Pharmacology Conference September 04-06, 2017 Paris, France; Pharmacovigilance Conferences 2018 USA, 3rd Conference on Clinical Research September 20-21, 2017 Dublin, Ireland; 9th Pharmaceutical Chemical Analysis Conference October 02-03, 2017 Vienna, Austria; 5th Conference on Pain Research and Management October 05-06, 2017 London, UK; 20th Conference on Biotechnology December 07-09, 2017 Madrid, Spain; 12th Pharma Industry Conference February 26- 27, 2018 London, UK; 16th Conference Novel Drug Delivery Systems March 19-21, 2018 Berlin, Germany; Pharmacovigilance Conferences 2018 Asia, 11th Biosimilars Conference April 26-27, 2018 Rome, Italy; 10th Pharma Conference May 07-09, 2018 Frankfurt, Germany; 4th Conference on Natural Products June 11-13, 2018 Rome, Italy
Related Associations or Societies:
American Association of Pharmaceutical Scientists, Indian Pharmaceutical Association, Association of Clinical Research, The International Society of Pharmacovigilance, Canadian Society of Pharmaceutical Sciences (CSPS), Association of Clinical Research Organization (ACRO)
Track 12: Case Report in Clinical Trials
Case Report in Clinical Trials plays a key role in Clinical Research. Case reports may be on unexpected association between diseases, disorders or symptoms. An unexpected event in the course of observing, treating a patient, possibilities of pathogenesis of a disease or an adverse effect. Unique or rare salient features of a disease or therapeutic approaches or a notable variation of the anatomical structures.
Related Pharmacovigilance Congress | Pharmacovigilance Events
3rd Conference on Clinical trials September 20-21 Dublin, Ireland; 5th Conference on Pharmacology September 25-26, 2017 Singapore; 8th Pharmaceutical Analysis Conference September 25-27,2017 Vienna, Austria
Related Associations or Societies:
The Society of Clinical Research Associates (SOCRA), Clinical Research Society, Society for Clinical Trials (SCT), Associates of Clinical Research, Association of British Pharmaceutical Industry, Canadian Society of Pharmaceutical Sciences (CSPS)
Track 13: Drug Safety
Drug Safety heavily focuses on adverse drug reactions which are defined as any response to a drug which is noxious and unintended, including lack of efficacy. Medication errors such as overdose, and misuse and abuse of a drug as well as drug exposure during pregnancy and breastfeeding, are also of interest, even without an adverse event, because they may result in an adverse drug reaction.
Information received from patients and healthcare providers via pharmacovigilance agreements (PVAs), as well as other sources such as the medical literature, plays a critical role in providing the data necessary for pharmacovigilance to take place. Ultimately, drug safety is concerned with identifying the hazards associated with pharmaceutical products and with minimizing the risk of any harm that may come to patients. Companies must conduct a comprehensive drug safety and pharmacovigilance audit to assess their compliance with worldwide laws, regulations, and guidance.
Related Pharmacovigilance Congress | Pharmacovigilance Events
9th Conference on Pharmacology September 04-06, 2017 Paris, France; Pharmacovigilance Conferences 2018, 3rd Conference on Clinical Research September 20-21, 2017 Dublin, Ireland; 9th Pharmaceutical Chemical Analysis Conference October 02-03, 2017 Vienna, Austria; 5th Pain Research and Management Conference October 05-06, 2017 London, UK; 20th Conference on Pharmaceutical Biotechnology December 07-09, 2017 Madrid, Spain; 12th Conference on Pharmaceutical Sciences February 26- 27, 2018 London, UK; 16th Conference on Pharmaceutics March 19-21, 2018 Berlin, Germany; Pharmacovigilance Conferences 2018 USA, 11th Biosimilars Conference April 26-27, 2018 Rome, Italy; Pharmacovigilance Conferences 2018 Asia, 10th Pharma Conference May 07-09, 2018 Frankfurt, Germany; 4th Conference on Marine Drugs June 11-13, 2018 Rome, Italy
Related Associations or Societies:
Society for Clinical Trials (SCT), Association of Clinical Research, The Society of Clinical Research Associates, Association of Clinical Research Organization, Clinical Research Society, Indian Society for Clinical Organization Research (ISCR)
Track 14: Clinical Data Base Management
There is an advantage in centralising all safety data, clinical data, analysis and reporting with one provider. Pharmacovigilance Software tool provides comprehensive analysis of adverse events arising from the use of Pharmaceutical products (Medicinal Product, Medical Device, Vaccines, Non-Drug Therapy and Veterinary Medicinal Product). The drug safety database allows the risk- benefit analysis of medicinal products taking into account new and emerging information, in the context of cumulative information. Pharmacovigilance since beginning has been a compliance driven activity, wherein your regulatory compliance determines company’s risk assessment scores. A drug safety database offers scheduling of alerts for expedited cases, follow-up cases and PSUR/PADER reports submission to meet regulatory timeline compliance.
Related Pharmacovigilance Congress | Pharmacovigilance Events
9th Conference on Pharmacology September 04-06, 2017 Paris, France; Pharmacovigilance Conferences 2018 Asia, 3rd Conference on Clinical trials September 20-21, 2017 Dublin, Ireland; 9th Pharmaceutical Chemical Analysis Conference October 02-03, 2017 Vienna, Austria; 5th Conference on Pain Research October 05-06, 2017 London, UK; Pharmacovigilance Conferences 2018 USA, 20th Conference on Biotechnology December 07-09, 2017 Madrid, Spain; 12th Conference on Pharmaceutical Sciences February 26- 27, 2018 London, UK; 16th Conference on Novel Drug Delivery March 19-21, 2018 Berlin, Germany; 11th Biosimilars Conference April 26-27, 2018 Rome, Italy; Pharmacovigilance Conferences 2018, 10th Pharma Conference May 07-09, 2018 Frankfurt, Germany; 4th Conference on Marine Drugs June 11-13, 2018 Rome, Italy
Related Associations or Societies:
The Society of Clinical Research Associates (SOCRA), Clinical Research Society, Society for Clinical Trials (SCT), Associates of Clinical Research, Association of British Pharmaceutical Industry, Canadian Society of Pharmaceutical Sciences (CSPS)
Track 15: PV Consulting’s and Business opportunity
Due to the changing resources necessary to fulfil the regulatory requirements, some companies also choose to outsource or out task regulatory affairs to external service providers. Regulatory Affairs department is constantly evolving and growing and is the one which is least impacted during the Acquisition and Merger, and also during recession. The global pharmacovigilance market and Business opportunity was valued at USD 2,408.0 million in 2013 and is expected to grow at a CAGR of 12.6% during the forecast period. Phase III clinical trials market was the second largest and was valued at over USD 750.0 million in 2013.
Related Pharmacovigilance Conferences| Pharmacovigilance Meetings
9th Conference on Pharmacology September 04-06, 2017 Paris, France; Pharmacovigilance Conferences 2018, 3rd Conference on Advanced Clinical Research September 20-21, 2017 Dublin, Ireland; 9th Pharmaceutical Chemical Analysis Conference October 02-03, 2017 Vienna, Austria; 5th Conference on Pain Research and Management October 05-06, 2017 London, UK; 20th Conference on Pharmaceutical December 07-09, 2017 Madrid, Spain; 12th Conference on Pharma Industry February 26- 27, 2018 London, UK; 16th Conference on Drug Delivery Systems March 19-21, 2018 Berlin, Germany; Pharmacovigilance Conferences 2018 USA, 11th Biosimilars Conference April 26-27, 2018 Rome, Italy; 10th Pharma Conference May 07-09, 2018 Frankfurt, Germany; Pharmacovigilance Conferences 2018 Asia, 4th Conference on Natural Products June 11-13, 2018 Rome, Italy
Related Associations or Societies:
Association of British Pharmaceutical Industry, Canadian Society of Pharmaceutical Sciences (CSPS), Pharmaceutical Information and Pharmacovgilance Association (PIPA), American Association of Pharmaceutical; Scientists, American Society of Pharmacognosy
Track 16: Regulatory Affairs
Regulatory Affairs for clinical trials is the major part in the clinical trials approaches. Every clinical trial must be analysed according to the Regulatory Affairs Guidelines. There are several Regulatory Affairs departments depending upon the countries within ever growing pace of companies. Global Harmonization in standards has led to consistent approach in regulatory submissions and hence its review.
Related Pharmacovigilance Congress | Pharmacovigilance Events
9th Conference on Pharmacology September 04-06, 2017 Paris, France; Pharmacovigilance Conferences 2018 Asia, 3rd Conference on Advanced Clinical Research September 20-21, 2017 Dublin, Ireland; 9th Pharmaceutical Chemical Analysis Conference October 02-03, 2017 Vienna, Austria; 5th Conference on Pain Research October 05-06, 2017 London, UK; 20th Conference on Biotechnology December 07-09, 2017 Madrid, Spain; 12th Conference on Pharmaceutical Sciences February 26- 27, 2018 London, UK; 16th Conference Novel Drug Delivery Systems March 19-21, 2018 Berlin, Germany; Pharmacovigilance Conferences 2018 USA, 11th Biosimilars Conference April 26-27, 2018 Rome, Italy; 10th Pharma Conference May 07-09, 2018 Frankfurt, Germany; Pharmacovigilance Conferences 2018 Asia, 4th Conference on Marine Drugs June 11-13, 2018 Rome, Italy
Related Associations or Societies:
The International Society of Pharmacovigilance (ISOP), Pharmaceutical Information and Pharmacovigilance Association (PIPA), Association of Clinical Research Organization (ACRO), Indian Pharmaceutical Association, Association of Clinical Research
Track 17: Entrepreneurs Investment Meet
Initial investment, capital requirements, business financing, current trends and the amount of time will take to get your business up and running. Pharmacovigilance 2017 focus in collaboration and communication among the Pharma Professionals, Business Entrepreneurs, CEO’s and Pharma Industrial Persons
We solicit your gracious presence at the upcoming 11th International Conference and Exhibition on Pharmacovigilance and Drug Safety scheduled to happen during June 21-22, 2018 in London, UK focussing on the advancements in emerging research and technologies in pharmacovigilance, clinical trials and risk management.
The field of Pharmacovigilance is growing rapidly and its development is making tremendous impacts in medical sciences and pharmaceuticals. Pharmacovigilance 2018 emphasizes on how the importance and significance can be gauged by the fact that it has made huge advancements over the course of time and is continuing to influence various sectors.
Theme: “Global Pharmacovigilance Approaches and Innovations for Patient Benefit Risk Management”
Track 1: Pre-Clinical and Clinical Trials
Clinical trials allow the drug to be tested for safety by different ethnic population. Due to the higher medical needs and increasing disease prevalence, developing countries are becoming a hub for clinical trial execution. The clinical trials market has been estimated to reach USD 14.2 billion in 2016 and is projected to reach around USD 22 billion by the year 2021, and the annual growth rate of (7.5%). There are additionally numerous clinical trials started by scholarly clinical scientists. Whether started by industry or by scholastic clinical examiners and research is frequently performed in national, European and overall consortia, which can be expansive ones. Clinical research brings up moral and security issues. Clinical research is exceedingly controlled. To encourage and coordinated efforts crosswise over fringes.
Related Pharmacovigilance Congress | Pharmacovigilance Events
9th Conference on Pharmacology during September 04-06, 2017 at Paris, France; 3rd Conference on Advanced Clinical Research September 20-21, 2017 Dublin, Ireland; 9th Pharmaceutical Chemical Analysis Conference October 02-03, 2017 Vienna, Austria; 5th Conference on Pain Research and Management October 05-06, 2017 London, UK; 20th Conference on Pharmaceutical Biotechnology December 07-09, 2017 Madrid, Spain; 12th Conference on Pharmaceutical Sciences February 26- 27, 2018 London, UK; 16th Conference on Pharmaceutics March 19-21, 2018 Berlin, Germany; 11th Biosimilars Conference April 26-27, 2018 Rome, Italy; 10th Pharma Conference May 07-09, 2018 Frankfurt, Germany; 4th Conference on Marine Drugs June 11-13, 2018 Rome, Italy
Related Associations or Societies:
Society for Clinical Trials (SCT), Association of Clinical Research, The Society of Clinical Research Associates, Association of Clinical Research Organization, Clinical Research Society, Indian Society for Clinical Organization Research (ISCR)
Track 2: Adverse Drug Reactions
Adverse drug reactions can be considered a form of toxicity or enhanced drug effects that occur during appropriate use (eg, when drug metabolism is temporarily inhibited by a disorder or another drug). In the US, 3 to 7% of all hospitalizations are due to adverse drug reactions. ADRs occur during 10 to 20% of hospitalizations; about 10 to 20% of these ADRs are severe. Incidence of death due to ADRs is unknown; suggested rates of 0.5 to 0.9% may be falsely high because many of the patients included had serious and complex disorders. Incidence and severity of adverse drug reactions vary by patient characteristics (eg, age, sex, ethnicity, coexisting disorders, genetic or geographic factors) and by drug factors (eg, type of drug, administration route, treatment duration, dosage, and bioavailability). Incidence is higher with advanced age and polypharmacy.
Related Pharmacovigilance Conferences | Pharmacovigilance Meetings
9th Conference on Pharmacology September 04-06, 2017 Paris, France; 3rd Clinical Trials Conference September 20-21, 2017 Dublin, Ireland; Pharmacovigilance Conferences 2018 Asia, 9th Conference on Pharmaceutical Chemical Analysis October 02-03, 2017 Vienna, Austria; 5th Conference on Pain Research and Management October 05-06, 2017 London, UK; Pharmacovigilance Conferences 2018, Conference on Pharmaceutical Biotechnology December 07-09, 2017 Madrid, Spain; 12th Conference on Pharmaceutical Sciences in Pharma Industry February 26-27, 2018 London, UK; 16th Conference on Drug Delivery Systems March 19-21, 2018 Berlin, Germany; 11th European Biosimilars Conference April 26-27, 2018 Rome, Italy; Pharmacovigilance Conferences 2018 Asia,10th Pharma Conference May 07-09, 2018 at Frankfurt, Germany; 4th Conference on Natural Products June 11-13, 2018 Rome, Italy
Related Associations or Societies:
American Association of Pharmaceutical Scientists, Indian Pharmaceutical Association, Association of Clinical Research, The International Society of Pharmacovigilance, Canadian Society of Pharmaceutical Sciences (CSPS), Association of Clinical Research Organization (ACRO)
Track 3: Pharmacovigilance and Risk Management
Pharmacovigilance and Risk Management comprises set of pharmacovigilance activities and interventions designed to identify, characterise, prevent or minimise risks relating to medicinal and therapeutic products including the assessment of the effectiveness of their clinical interventions and combination therapies. Drug industry need to promote companies in pharmacovigilance practice to use information technology and to review softwares used in pharmacovigilance and clinical trials. Monitoring unlicensed, off labels and orphan drugs is major task in risk management. Many experts from different pharmacovigilance CRO's , pharmacovigilance service providers and industrial delegates are participating in this conference to share their knowledge and discuss about the new updates.
Related Pharmacovigilance Conferences | Pharmacovigilance Meetings
9th Pharmacology Conference September 04-06, 2017 Paris, France; 3rd Conference on Clinical Research September 20-21, 2017 Dublin, Ireland; Pharmacovigilance Conferences 2018 Asia, 9th Pharmaceutical Analysis Conference October 02-03, 2017 at Vienna, Austria; 5th Conference on Pain Research October 05-06, 2017 London, UK; Pharmaceutical Biotechnology Conference December 07-09, 2017 Madrid, Spain; Pharmaceutical Sciences Conference February 26- 27, 2018 London, UK; Pharmacovigilance Conferences 2018, 16th Novel Drug Delivery Systems Conference March 19-21, 2018 Berlin, Germany; Pharmacovigilance Conferences 2018 USA, 11th Biosimilars Conference April 26-27, 2018 Rome, Italy; 10th Pharma Conference May 07-09, 2018 Frankfurt, Germany; 4th Conference on Marine Drugs June 11-13, 2018 Rome, Italy
Related Associations or Societies:
The International Society of Pharmacovigilance (ISOP), Pharmaceutical Information and Pharmacovigilance Association (PIPA), Association of Clinical Research Organization (ACRO), Indian Pharmaceutical Association, Association of Clinical Research
Track 4: Good Pharmacovigilance Practice
The role of Good Pharmacovigilance Practice and Pharmacoepidemiology in Risk Management is mainly to increase the probability of beneficial effects of a drug in a population than the probability of adverse effects and to maintain the Good Reporting Practices by avoiding the major problems in risk management. Also it is important to concentrate on Signal investigation by gathering the information on new or unknown drug effects that is potentially caused by a medicine and that finally should lead to ensuring safety. The pharmacovigilance and clinical trials services providing companies should have the Pharmacovigilance certification.
Related Pharmacovigilance Conferences| Pharmacovigilance Meetings
9th Pharmacology Conference September 04-06, 2017 Paris, France; 3rd Conference on Clinical trials September 20-21, 2017 Dublin, Ireland; Pharmacovigilance Conferences 2018, 9th Pharmaceutical Chemical Analysis Conference October 02-03, 2017 Vienna, Austria; 5th Pain Research Conference October 05-06, 2017 London, Pharmacovigilance Conferences 2018 USA, UK; Pharmaceutical Biotechnology Conference December 07-09, 2017 Madrid, Spain; Pharmaceutical Sciences in Pharma Industry Conference February 26- 27, 2018 London, UK; Pharmaceutics and Drug Delivery Systems Conference March 19-21, 2018 Berlin, Germany; 11th Biosimilars Conference April 26-27, 2018 Rome, Italy; 10th European Pharma Conference May 07-09, 2018 Frankfurt, Germany; Pharmacovigilance Conferences 2018 Asia, 4th Conference on Natural Products June 11-13, 2018 Rome, Italy
Related Associations or Societies:
American Association of Pharmaceutical Scientists, Indian Pharmaceutical Association, Association of Clinical Research, The International Society of Pharmacovigilance, Canadian Society of Pharmaceutical Sciences (CSPS), Association of Clinical Research Organization (ACRO)
Track 5: Pharmacy Practices and its Challenges
Pharmacy practice is the field of pharmacy which involves developing the professional roles of pharmacists. It includes Disease-state management, Clinical drug interventions, Pharmacy professional development and pharmaceutical care, pharmaceutical compounding and health psychology, patient care, drug abuse prevention, prevention of drug interactions or minimisation of adverse events and drug incompatibility and community pharmacy.
Related Pharmacovigilance Conferences| Pharmacovigilance Meetings
5th Pharmaceutical Sciences Conference; 8th Conference on Pharmacology, August 07-09, 2017, Pharmacovigilance Conferences 2018, Paris, France; 3rd Conference on Clinical Research September 20-21 Dublin, Ireland; Pharmacovigilance Conferences 2018 USA, 8th Pharmaceutical Analysis Conference September 25-27, 2017 at Vienna, Austria
Related Associations or Societies:
The Society of Clinical Research Associates (SOCRA), Clinical Research Society, Society for Clinical Trials (SCT), Associates of Clinical Research, Association of British Pharmaceutical Industry, Canadian Society of Pharmaceutical Sciences (CSPS)
Track 6: Biopharmaceutical Sciences
In Clinical Pharmacology and Biopharmaceutics track we will discuss about the Rational drug management of cancer, diabetes and cardiovascular disorders, and Management of psychiatric disorders and autoimmune disorders. Along with clinical trials Bioavailability and bioequivalence studies also plays major role in clinical research.The global market for Biopharmaceutics in 2013 was $305.1 million, which is expected to reach about $326.3 million by year-end 2014. The projected PAT instrumentation market is expected to be valued at around $450.6 million by 2019 at a compound annual growth rate (CAGR) of 6.7% for the period of 2014 to 2019.
Related Pharmacovigilance Conferences| Pharmacovigilance Meetings
9th Pharmacology Conference September 04-06, 2017 Paris, France; 3rd Clinical trials Conference September 20-21, 2017 Dublin, Ireland; Pharmacovigilance Conferences 2018 Asia, 9th Chemical Analysis Conference October 02-03, 2017 Vienna, Austria; 5th Conference on Pain Research October 05-06, 2017 London, UK; 20th Conference on Biotechnology December 07-09, 2017 Madrid, Spain; Pharma Industry Conference February 26- 27, 2018 London, UK; 16th Drug Delivery Conference March 19-21, 2018 Berlin, Germany; 11th Biosimilars Conference April 26-27, 2018 Rome, Italy; Pharmacovigilance Conferences 2018 USA, 10th Conference on Pharma May 07-09, 2018 Frankfurt, Germany; 4th Conference on Drugs and Natural Products June 11-13, 2018 Rome, Italy
Related Associations or Societies:
Association of British Pharmaceutical Industry, Canadian Society of Pharmaceutical Sciences (CSPS), Pharmaceutical Information and Pharmacovigilance Association (PIPA), American Association of Pharmaceutical; Scientists, American Society of Pharmacognosy
Track 7: Clinical Trials on Various Disorders
Clinical Trials on various diseases include Clinical Trials in long chronic diseases like AIDS, Benign and Malignant Tumours, Cardiovascular diseases, Diabetes, Clinical Trials on Monoclonal and Polyclonal Antibodies, Neurological, Physiological and Psychological Disorders
Related Pharmacovigilance Congress | Pharmacovigilance Events
9th Pharmacology Conference September 04-06, 2017 Paris, France; Pharmacovigilance Conferences 2018, 3rd Clinical trials Conference September 20-21, 2017 Dublin, Ireland; 9th Pharmaceutical Chemical Analysis Conference October 02-03, 2017 Vienna, Austria; 5th Conference on Pain Research and Management October 05-06, 2017 London, UK; Pharmacovigilance Conferences 2018 USA, 20th Pharmaceutical Biotechnology Conference December 07-09, 2017 Madrid, Spain; 12th Conference on Sciences February 26- 27, 2018 London, UK; 16th Pharmaceutics and Novel Drug Delivery Systems Conference March 19-21, 2018 Berlin, Germany; 11th Biosimilars Conference April 26-27, 2018 Rome, Italy; Pharmacovigilance Conferences 2018 Asia, 10th Pharma Conference May 07-09, 2018 Frankfurt, Germany; 4th Natural Products Conference June 11-13, 2018 Rome, Italy
Related Associations or Societies:
The Society of Clinical Research Associates (SOCRA), Clinical Research Society, Society for Clinical Trials (SCT), Associates of Clinical Research, Association of British Pharmaceutical Industry, Canadian Society of Pharmaceutical Sciences (CSPS)
Track 8: Data Quality Management and Analysis
Good data quality management in pharmacovigilance can be relied only on information gathered from the collection of individual case safety reports and other pharmacoepidemiological data. Quality management consists of quality planning, quality control, quality assurance and quality improvements. The pharmacovigilance data processing cycle starts with data collection and, in computerised systems, data entry; the next step is data storage and maintenance; followed by data selection, retrieval and manipulation. The resulting data output is analysed and assessed. Finally, conclusions are drawn and decisions are made. The quality of a pharmacovigilance data system can be defined as a measure of excellence or a state of being free from defects, deficiencies and significant variations and the data quality management includes all the activities that organizations use to direct, control and coordinate the quality of data.
Related Pharmacovigilance Congress | Pharmacovigilance Events
9th Conference on Pharmacology September 04-06, 2017 Paris, France; Pharmacovigilance Conferences 2018, 3rd Clinical Research Conference September 20-21, 2017 Dublin, Ireland; 9th Pharmaceutical Chemical Analysis Conference October 02-03, 2017 Vienna, Austria; 5th Conference on Pain Research October 05-06, 2017 London, UK; Pharmacovigilance Conferences 2018 USA, 20th Conference on Pharmaceutical Biotechnology December 07-09, 2017 Madrid, Spain; 12th Congress on Pharmaceutical Sciences and in Pharma Industry Conference February 26- 27, 2018 London, UK; 16th Drug Delivery Systems Conference March 19-21, 2018 Berlin, Germany; 11th Biosimilars Conference April 26-27, 2018 Rome, Italy; 10th Pharma Conference May 07-09, 2018 Frankfurt, Germany; Pharmacovigilance Conferences 2018 Asia, 4th Conference on Marine Drugs June 11-13, 2018 Rome, Italy
Related Associations or Societies:
Pharmaceutical Information and Pharmacovgilance Association (PIPA), American Association of Pharmaceutical; Scientists, American Society of Pharmacognosy , Association of Clinical Research Organization (ACRO), Society for Clinical Trials (SCT), Association of Clinical Research
Track 9: Pharmacovigilance Significance & Scope
Concept of Pharmacovigilance and its Significance enhances the impact of pharmacovigilance on patient welfare and public health and to know what is pharmacovigilance. This track gives a brief discussion on Pharmacovigilance role in healthcare system. Pharmacovigilance legislation gives an outlook on the rules and laws to follow in Pharmacovigilance practice. The Role of pharma industries in the improvement of pharmacovigilance system is very crucial to maintain the safety data, Detection and Evaluation of drug safety signals through manual and medical devices reporting. Pharmacovigilance scope also deals as Ecopharmacovigilance (EPV), pharmacoenvironmentology and pharmacovigilance in herbal medicines.
Related Pharmacovigilance Conferences| Pharmacovigilance Meetings
9th Pharmacology Conference September 04-06, 2017 Paris, France; Pharmacovigilance Conferences 2018 Asia, 3rd Advanced Clinical Research Conference September 20-21, 2017 Dublin, Ireland; 9th Pharmaceutical Analysis Conference October 02-03, 2017 Vienna, Austria; 5th Pain Research Conference October 05-06, 2017 London, UK; Pharmacovigilance Conferences 2018 USA, 20th Pharmaceutical Biotechnology Conference December 07-09, 2017 Madrid, Spain; 12th Conference on Pharma Industry February 26- 27, 2018 London, UK; 16th Conference on Novel Drug Delivery March 19-21, 2018 Berlin, Germany; 11th Biosimilars Conference April 26-27, 2018 Rome, Italy; 10th Pharma Conference May 07-09, 2018 Frankfurt, Germany; Pharmacovigilance Conferences 2018, 4th Conference on Marine Drugs June 11-13, 2018 Rome, Italy
Related Associations or Societies:
American Association of Pharmaceutical Scientists, Indian Pharmaceutical Association, Association of Clinical Research, The International Society of Pharmacovigilance, Canadian Society of Pharmaceutical Sciences (CSPS), Association of Clinical Research Organization (ACRO)
Track 10: Diversity in Industrial Clinical Trials and Clinical Research
The clinical trial industry is constantly evolving with diversified clinical research technologies and new clinical studies are being launched at an ever-growing pace. Clinical trials have always been a vital part of the medicine development process, as they provide clinical data on the best ways for treating pathological disorders and diseases. The importance of diversity in industrial clinical trials and clinical research is to ensure that industrial clinical trials are doing due diligence and being as strategic as possible in their results. This diversity makes us to better understand the unmet medical needs of patients.
Related Pharmacovigilance Congress | Pharmacovigilance Events
9th Conference on Pharmacology September 04-06, 2017 Paris, France; Pharmacovigilance Conferences 2018, 3rd Conference on Clinical Research September 20-21, 2017 Dublin, Ireland; 9th Pharmaceutical Analysis Conference October 02-03, 2017 Vienna, Austria; 5th Conference on Pain Research October 05-06, 2017 London, UK; Conference on Biotechnology December 07-09, 2017 Madrid, Spain; 12th Pharmaceutical Sciences Conference February 26- 27, 2018 London, UK; 16th Conference on Pharmaceutics March 19-21, 2018 Berlin, Germany; Pharmacovigilance Conferences 2018 USA, 11th Biosimilars Conference April 26-27, 2018 Rome, Italy; 10th Pharma Conference May 07-09, 2018 Frankfurt, Germany; Pharmacovigilance Conferences 2018 Asia, 4th Conference on Marine Drugs June 11-13, 2018 Rome, Italy
Related Associations or Societies:
Society for Clinical Trials (SCT), Association of Clinical Research, The Society of Clinical Research Associates, Association of Clinical Research Organization, Clinical Research Society, Indian Society for Clinical Organization Research (ISCR)
Track 11: Clinical Research and Statistics
In Clinical Research, Statistics plays a prominent role in regulatory submissions. Statistical analysis of Pharmacovigilance can be achieved by several guidelines mainly ICH guidelines. Adverse Drug Reactions reports can also be considered for the regulatory submission.
Related Pharmacovigilance Congress | Pharmacovigilance Events
9th Pharmacology Conference September 04-06, 2017 Paris, France; Pharmacovigilance Conferences 2018 USA, 3rd Conference on Clinical Research September 20-21, 2017 Dublin, Ireland; 9th Pharmaceutical Chemical Analysis Conference October 02-03, 2017 Vienna, Austria; 5th Conference on Pain Research and Management October 05-06, 2017 London, UK; 20th Conference on Biotechnology December 07-09, 2017 Madrid, Spain; 12th Pharma Industry Conference February 26- 27, 2018 London, UK; 16th Conference Novel Drug Delivery Systems March 19-21, 2018 Berlin, Germany; Pharmacovigilance Conferences 2018 Asia, 11th Biosimilars Conference April 26-27, 2018 Rome, Italy; 10th Pharma Conference May 07-09, 2018 Frankfurt, Germany; 4th Conference on Natural Products June 11-13, 2018 Rome, Italy
Related Associations or Societies:
American Association of Pharmaceutical Scientists, Indian Pharmaceutical Association, Association of Clinical Research, The International Society of Pharmacovigilance, Canadian Society of Pharmaceutical Sciences (CSPS), Association of Clinical Research Organization (ACRO)
Track 12: Case Report in Clinical Trials
Case Report in Clinical Trials plays a key role in Clinical Research. Case reports may be on unexpected association between diseases, disorders or symptoms. An unexpected event in the course of observing, treating a patient, possibilities of pathogenesis of a disease or an adverse effect. Unique or rare salient features of a disease or therapeutic approaches or a notable variation of the anatomical structures.
Related Pharmacovigilance Congress | Pharmacovigilance Events
3rd Conference on Clinical trials September 20-21 Dublin, Ireland; 5th Conference on Pharmacology September 25-26, 2017 Singapore; 8th Pharmaceutical Analysis Conference September 25-27,2017 Vienna, Austria
Related Associations or Societies:
The Society of Clinical Research Associates (SOCRA), Clinical Research Society, Society for Clinical Trials (SCT), Associates of Clinical Research, Association of British Pharmaceutical Industry, Canadian Society of Pharmaceutical Sciences (CSPS)
Track 13: Drug Safety
Drug Safety heavily focuses on adverse drug reactions which are defined as any response to a drug which is noxious and unintended, including lack of efficacy. Medication errors such as overdose, and misuse and abuse of a drug as well as drug exposure during pregnancy and breastfeeding, are also of interest, even without an adverse event, because they may result in an adverse drug reaction.
Information received from patients and healthcare providers via pharmacovigilance agreements (PVAs), as well as other sources such as the medical literature, plays a critical role in providing the data necessary for pharmacovigilance to take place. Ultimately, drug safety is concerned with identifying the hazards associated with pharmaceutical products and with minimizing the risk of any harm that may come to patients. Companies must conduct a comprehensive drug safety and pharmacovigilance audit to assess their compliance with worldwide laws, regulations, and guidance.
Related Pharmacovigilance Congress | Pharmacovigilance Events
9th Conference on Pharmacology September 04-06, 2017 Paris, France; Pharmacovigilance Conferences 2018, 3rd Conference on Clinical Research September 20-21, 2017 Dublin, Ireland; 9th Pharmaceutical Chemical Analysis Conference October 02-03, 2017 Vienna, Austria; 5th Pain Research and Management Conference October 05-06, 2017 London, UK; 20th Conference on Pharmaceutical Biotechnology December 07-09, 2017 Madrid, Spain; 12th Conference on Pharmaceutical Sciences February 26- 27, 2018 London, UK; 16th Conference on Pharmaceutics March 19-21, 2018 Berlin, Germany; Pharmacovigilance Conferences 2018 USA, 11th Biosimilars Conference April 26-27, 2018 Rome, Italy; Pharmacovigilance Conferences 2018 Asia, 10th Pharma Conference May 07-09, 2018 Frankfurt, Germany; 4th Conference on Marine Drugs June 11-13, 2018 Rome, Italy
Related Associations or Societies:
Society for Clinical Trials (SCT), Association of Clinical Research, The Society of Clinical Research Associates, Association of Clinical Research Organization, Clinical Research Society, Indian Society for Clinical Organization Research (ISCR)
Track 14: Clinical Data Base Management
There is an advantage in centralising all safety data, clinical data, analysis and reporting with one provider. Pharmacovigilance Software tool provides comprehensive analysis of adverse events arising from the use of Pharmaceutical products (Medicinal Product, Medical Device, Vaccines, Non-Drug Therapy and Veterinary Medicinal Product). The drug safety database allows the risk- benefit analysis of medicinal products taking into account new and emerging information, in the context of cumulative information. Pharmacovigilance since beginning has been a compliance driven activity, wherein your regulatory compliance determines company’s risk assessment scores. A drug safety database offers scheduling of alerts for expedited cases, follow-up cases and PSUR/PADER reports submission to meet regulatory timeline compliance.
Related Pharmacovigilance Congress | Pharmacovigilance Events
9th Conference on Pharmacology September 04-06, 2017 Paris, France; Pharmacovigilance Conferences 2018 Asia, 3rd Conference on Clinical trials September 20-21, 2017 Dublin, Ireland; 9th Pharmaceutical Chemical Analysis Conference October 02-03, 2017 Vienna, Austria; 5th Conference on Pain Research October 05-06, 2017 London, UK; Pharmacovigilance Conferences 2018 USA, 20th Conference on Biotechnology December 07-09, 2017 Madrid, Spain; 12th Conference on Pharmaceutical Sciences February 26- 27, 2018 London, UK; 16th Conference on Novel Drug Delivery March 19-21, 2018 Berlin, Germany; 11th Biosimilars Conference April 26-27, 2018 Rome, Italy; Pharmacovigilance Conferences 2018, 10th Pharma Conference May 07-09, 2018 Frankfurt, Germany; 4th Conference on Marine Drugs June 11-13, 2018 Rome, Italy
Related Associations or Societies:
The Society of Clinical Research Associates (SOCRA), Clinical Research Society, Society for Clinical Trials (SCT), Associates of Clinical Research, Association of British Pharmaceutical Industry, Canadian Society of Pharmaceutical Sciences (CSPS)
Track 15: PV Consulting’s and Business opportunity
Due to the changing resources necessary to fulfil the regulatory requirements, some companies also choose to outsource or out task regulatory affairs to external service providers. Regulatory Affairs department is constantly evolving and growing and is the one which is least impacted during the Acquisition and Merger, and also during recession. The global pharmacovigilance market and Business opportunity was valued at USD 2,408.0 million in 2013 and is expected to grow at a CAGR of 12.6% during the forecast period. Phase III clinical trials market was the second largest and was valued at over USD 750.0 million in 2013.
Related Pharmacovigilance Conferences| Pharmacovigilance Meetings
9th Conference on Pharmacology September 04-06, 2017 Paris, France; Pharmacovigilance Conferences 2018, 3rd Conference on Advanced Clinical Research September 20-21, 2017 Dublin, Ireland; 9th Pharmaceutical Chemical Analysis Conference October 02-03, 2017 Vienna, Austria; 5th Conference on Pain Research and Management October 05-06, 2017 London, UK; 20th Conference on Pharmaceutical December 07-09, 2017 Madrid, Spain; 12th Conference on Pharma Industry February 26- 27, 2018 London, UK; 16th Conference on Drug Delivery Systems March 19-21, 2018 Berlin, Germany; Pharmacovigilance Conferences 2018 USA, 11th Biosimilars Conference April 26-27, 2018 Rome, Italy; 10th Pharma Conference May 07-09, 2018 Frankfurt, Germany; Pharmacovigilance Conferences 2018 Asia, 4th Conference on Natural Products June 11-13, 2018 Rome, Italy
Related Associations or Societies:
Association of British Pharmaceutical Industry, Canadian Society of Pharmaceutical Sciences (CSPS), Pharmaceutical Information and Pharmacovgilance Association (PIPA), American Association of Pharmaceutical; Scientists, American Society of Pharmacognosy
Track 16: Regulatory Affairs
Regulatory Affairs for clinical trials is the major part in the clinical trials approaches. Every clinical trial must be analysed according to the Regulatory Affairs Guidelines. There are several Regulatory Affairs departments depending upon the countries within ever growing pace of companies. Global Harmonization in standards has led to consistent approach in regulatory submissions and hence its review.
Related Pharmacovigilance Congress | Pharmacovigilance Events
9th Conference on Pharmacology September 04-06, 2017 Paris, France; Pharmacovigilance Conferences 2018 Asia, 3rd Conference on Advanced Clinical Research September 20-21, 2017 Dublin, Ireland; 9th Pharmaceutical Chemical Analysis Conference October 02-03, 2017 Vienna, Austria; 5th Conference on Pain Research October 05-06, 2017 London, UK; 20th Conference on Biotechnology December 07-09, 2017 Madrid, Spain; 12th Conference on Pharmaceutical Sciences February 26- 27, 2018 London, UK; 16th Conference Novel Drug Delivery Systems March 19-21, 2018 Berlin, Germany; Pharmacovigilance Conferences 2018 USA, 11th Biosimilars Conference April 26-27, 2018 Rome, Italy; 10th Pharma Conference May 07-09, 2018 Frankfurt, Germany; Pharmacovigilance Conferences 2018 Asia, 4th Conference on Marine Drugs June 11-13, 2018 Rome, Italy
Related Associations or Societies:
The International Society of Pharmacovigilance (ISOP), Pharmaceutical Information and Pharmacovigilance Association (PIPA), Association of Clinical Research Organization (ACRO), Indian Pharmaceutical Association, Association of Clinical Research
Track 17: Entrepreneurs Investment Meet
Initial investment, capital requirements, business financing, current trends and the amount of time will take to get your business up and running. Pharmacovigilance 2017 focus in collaboration and communication among the Pharma Professionals, Business Entrepreneurs, CEO’s and Pharma Industrial Persons
Conference Series is organizing splendorous Pharmaceutical conferences welcomes you to attend the 11th International Conference and Exhibition on Pharmacovigilance & Drug Safety to be held during June 21-22, 2018 in London, UK. It focuses on the advancements in Pharmacovigilance, Risk Management, Drug Safety.
The field of Pharmacovigilance is growing rapidly and its development is making tremendous impacts in medical sciences and pharmaceuticals. 11th International Conference and Exhibition on Pharmacovigilance & Drug Safety emphasizes on how the importance and significance can be gauged by the fact that it has made huge advancements over the course of time and is continuing to influence various sectors.
Why to attend???
With members from around the world focused on learning about Pharmacovigilance and its advances; this is your best opportunity to reach the largest assemblage of participants from the Pharmacovigilance community. Conduct presentations, distribute information, meet with current and potential scientists, make a splash with new drug developments, and receive named recognition at this 3-days event. World-Renowned Speakers, the most recent techniques, developments, and the newest updates in Pharmacovigilance are Hallmarks of this Conference.
Target Audience:
- Pharmacovigilance Students, Scientists
- Pharmacovigilance Researchers
- Pharmacovigilance Faculty
- Medical Colleges
- Pharmacovigilance Associations and Societies
- Business Entrepreneurs
- Training Institutes
- Software Developing Companies
- Manufacturing Medical Devices Companies
- Data Management Companies
Pharmacovigilance 2018 welcomes attendees, presenters, and exhibitors from all over the world to Munich, Germany. We are delighted to invite you all to attend and register for the “11th International Conference and Exhibition on Pharmacovigilance & Drug Safety” which is going to be held during June 21-22, 2018 in London, UK. The Organizing Committee is gearing up for an exciting and informative conference program including plenary lectures, symposia, workshops on a variety of topics, poster presentations and various programs for participants from all over the world. We invite you to join us at the Pharmacovigilance 2018, where you will be sure to have a meaningful experience with scholars from around the world. All the members of Pharmacovigilance 2018 Organizing Committee will look forward to meet you at Munich, Germany.
For more details please visit- http://market-analysis.conferenceseries.com/pharmacovigilance-market-reports
Importance and Scope: Pharmacovigilance is the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other medicine-related problem. The main purpose of pharmacovigilance is to improve the patient's safety and enhance his care in terms of the use of medicines, including paramedical interventions. Pharmacovigilance also supports public health programs by providing reliable information for the efficient assessment of the risk-benefit profile of medicines, contribute to the assessment of benefits, uses, side effects, harm, effectiveness and risk of medicines, encouraging the safe, rational and more effective (including cost-effective) use of various medicines. Promote education, understanding and clinical training in Pharmacovigilance and its effective availability to the public.
Why Munich, Germany?
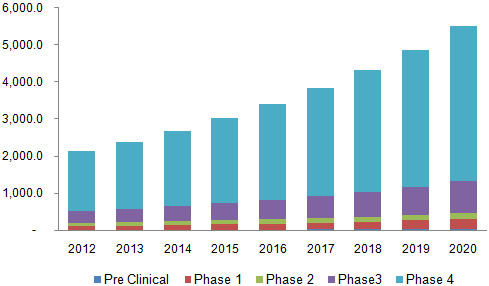
The Global Pharmacovigilance Market is expected to reach USD 5.51 billion by 2020. Increasing incidence rates of adverse drug reaction (ADR) and the introduction of stringent drug safety regulations are some key drivers of this market. ADR is responsible for approximately 5% of the hospitalization in developed countries annually, and this is expected to boost usage rates over the next six years. Pharmacovigilance has witnessed a significant rise in usage rates in the recent times owing to growing global geriatric population triggering a growth in demand for new drug development. Additionally, health regulatory authorities such as the U.S. FDA and EMEA (European Medicines Agency) are now emphasizing on electronic submission of data which is also expected to drive the pharmacovigilance market.
Pharmacovigilance Market, by Phases of Drug Development:
- Preclinical Studies
- Phase I
- Phase II
- Phase III
- Phase IV or Post Marketing Surveillance
Pharmacovigilance Market, by Type of Methods:
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
Pharmacovigilance Market, by Type of Service:
- In-House
- Contract Outsourcing
Conference Highlights
- Drug Safety
- Adverse Drug Reactions
- Pharmacovigilance Significance & Scope
- Good Pharmacovigilance Practice
- Pharmacovigilance and Risk Management
- Pharmacokinetics and Pharmacodynamics
- Pre-Clinical and Clinical Trials
- Clinical Trials on Various Disorders
- Diversity in Industrial Clinical Trials and Clinical Research
- Clinical Research and Statistics
- Case Report in Clinical Trial
- Data Quality Management and Analysis
- PV Data Base Managemaent
- PV Consultings And Bussiness Opportunity
- Regulatory Affairs
- Growth Strategies in Pharma
- Pharmacy Practices and its Challenges
- Entrepreneurs Investment Meet
Global pharmacovigilance market, by clinical trials, 2012 – 2020 (USD Million)
Conference Highlights
- Pre-Clinical and Clinical Trials
- Adverse Drug Reactions
- Pharmacovigilance and Risk Management
- Good Pharmacovigilance Practice
- Pharmacy Practices and its Challenges
- Biopharmaceutical Sciences
- Clinical Trials on Various Disorders
- Data Quality Management and Analysis
- Pharmacovigilance Significance & Scope
- Diversity in Industrial Clinical Trials and Clinical Research
- Clinical Research and Statistics
- Case Report in Clinical Trials
- Drug Safety
- Clinical Data Base Management
- PV Consultings and Business Opportunity
- Regulatory Affairs
- Entrepreneurs Investment Meet
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | June 21-22, 2018 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | Day 2 | |
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Pharmacovigilance
- Journal of Clinical & Experimental Pharmacology
- Journal of Clinical Trials
Abstracts will be provided with Digital Object Identifier by

























































































