Day 1 :
Keynote Forum
Maria Amparo Lopez Ruiz
Cardenal Herrera University, Spain
Keynote: Single oral ibuprofen overdose as cause of gastric hemorrhagic ulcer: A medication error in children
Time : 10:50-11:10

Biography:
Maria Amparo Lopez Ruiz has completed his PhD from Valencia University and postdoctoral studies from CEU Cardenal Herrera Health Sciences Faculty. She obtainedher doctorate in Medicine with the doctoral thesis on “Analysis of the use of medication in the paediatric population that visit accident and emergency department” with summa cum laude. She has achieved the qualification of “University Expert in Neonatology” from the Catholic University in Valencia and “master’s degree in Neonatology (from de SENeo -Neonatology Spanish Society-)”. She is medicine degree coordinator in CEU Cardenal Herrera University since 2015. She is the director of the “Master’s degree in Neonatal Intensive care and Neonatal Nursing”. She has attended to International Congresses of Pediatrics as a keynote speaker and she has been part of the Organizing Committee member for the 12th International Conference on pediatric pathology and Laboratory Medicine, and she has published in reputed international journals.
Abstract:
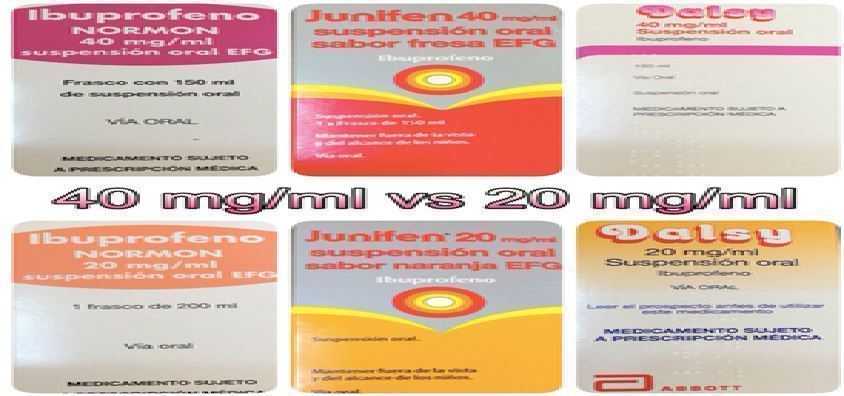
It has been established for a long time that non-steroidal anti-inflammatory drugs (NSAIDs) can produce gastrointestinal hemorrhagic ulcers when administered in high doses over a continuous period of time as in man- agement of chronic pain in the elderly, but it is not clear what effect NSAIDs have on paediatric gastric bleeding, since the use is usually for a short period of time. The use of NSAIDs among febrile pediatric patients has become a common treatment even without medical prescription, and is increasingly used alongside paracetamol (acetaminophen). A four-year-old girl was admitted to our hospital with a febrile status and hematemesis that started an hour earlier. On admission, she presented with tachycardia and fever, with normal blood pressure and a sore throat that started the previous day. The girl had received, several hours prior to admission, a single oral dose of ibu- profen that represented an increment of 50% over the indicated dosage for her weight. She had received no further treatment. The clinical history was of no relevance and the throat exploration showed some erythema and inflammation but not pus. The anamnesis revealed that a high ibuprofen dose had been administered by mistake. The digestive endoscopy found a gastrointestinal haemorrhagic ulcer that was still bleeding. After supressing the drug, conducting rehydration therapy, and administering intravenous ranitidine, progres- sion was favourable and the girl was discharged on the fourth day of admission. A single overdose, due to the similarity of the packaging of different concentration of the same active principle ingredient available in our country (Spain) can produce gastric bleeding. Combined treatment with NSAIDs and acetaminophen
Fig: Different percentage of quantities of the brands of ibuprofen on sell in Spain
Keynote Forum
Essam Ghanem
Celyad Biopharmaceutical, Belgium
Keynote: Driven strategy for effective monitoring pharmacovigilance quality system – key points
Time : 10:00-10:40

Biography:
Essam Ghanem is an experienced physician and qualified person for Pharmacovigilance with almost 34 years of experience in clinical research and drug development in academic institutes, pharmaceutical industry and clinical research organizations. He has around eight years of working experience as EUQPPV and as Consultant Safety Physician in the Pharmaceutical Industry. At present, he is the chief executive officer of the Pharmacovigilance Consultation Company VIGI-CARE BVBA and working as the head of drug safety and Pharmacovigilance at Celyad Biopharmaceutical, Belgium. He is a member of European society of CRO Federation (EUCROF) Working Group (PVWG)
Abstract:
Risk mitigation during medicinal products life cycle requires harmonization of pharmacovigilance activities handled by the involved stakeholders regarding their engagement strategy to ensure compliance. Pharmacovigilance system compliance necessitates the presence of effective quality system management. Real world data indicates that compliance rate needs to be optimized via incorporated standardized parameters and well identified compliance metrics. This presentation will provide an overview of the needed strategy for building up and monitoring of the quality system of pharmacovigilance. Such driven strategy for pharmacovigilance quality management will improves compliance rate and minimize authorities’ inspections critics.
Keynote Forum
Meital Simhi
Ben Gurion University of the Negev, Israel
Keynote: Mental health treatment preferences among Israeli postpartum mothers
Time : 09:30-10:15

Biography:
Abstract:
- Pre-Clinical and Clinical Trials | Adverse Drug Reactions | Pharmacovigilance and Risk Management | Good Pharmacovigilance Practice | Pharmacy Practices and its Challenges
Location: Atlantis 2
Session Introduction
Aaron Damien Barzey
ADB Medical, UK
Title: The possible effect of Brexit on the pharmaceutical industry: Pharmacovigilance
Time : 11:40-12:10

Biography:
Aaron Damien Barzey was the Global Labelling lead for the orphan drug ‘ofatumumab’. He was responsible for the company core datasheets, labelling strategy, EU labelling negations and oversaw the product launch in emerging markets. The major accomplishment was leading the launch of Arzerra for the treatment of chronic lymphatic leukaemia across the EU, Australia and other countries. In 2015 Aaron started his own regulatory consultancy, ADB Medical, providing ad-hoc support or project specific guidance to various companies. In 2016 Aaron was chosen as the pharmaceutical industry SME to discuss the possible impact of Brexit on the pharmaceutical industry, which included debating with Nigel Farage live on national television and to discuss further on live on UK TV with Piers Morgan and Susanna Reid.
Abstract:
The European Union (EU) has led the way in pharmacovigilance legislation, processes and understanding for many year and are the global leader in the review and approval of Biosimilars medicines, orphan drugs and paediatric medicines via the London based European Medicines Agency (EMA). Other countries, such as Australia, work side by side with the regulations and guidance produced from the EMA, and institutions, such as the World Health Organisation, have periodic meetings in London to discuss medicines. The United Kingdom is due to leave the EU on 29th March 2019 and the EMA and all its staff will have to relocate to remain in the EU as a result. All centralised registered products will need to be re-registered to one of the remaining 27 countries and there is the very real risk that this relocation will see losses in talented staff as well as hinder the approval and maintenance of innovative medicines and the maintenance of the current pharmacovigilance reporting mechanisms.
Sanjeev Miglani,
APCER Life Sciences, India
Title: Evaluation of signal detection methods in pharmacovigilance
Time : 12:10-12:40

Biography:
Sanjeev Miglani is an MD in internal medicine and has over 18 years of experience in the field of medicine, Pharmacovigilance and clinical research. He is the Vice President of Safety and Medical Affairs at APCER. Just before joining APCER, Sanjeev was the vice president for Pharmacovigilance and medical writing at Accenture. before joining Accenture, Sanjeev was the COO Officer and Scientific Director of CIDP Biotech. Prior to CIDP, Sanjeev was the Director of Medical Affairs at CliniRx. Before CliniRx, He has worked with Ranbaxy as a senior manager in the department of medical affairs and pharmacovigilance. He has also worked as a senior resident in cardiology and medicine department in some of the prestigious hospitals of New Delhi. He is a life time member of API and a fellow of Indian Association of Clinical medicine. He has numerous publications in medical journals and books to his credit.
Abstract:
The pharmaceutical companies collect the adverse events (AE) data from varied sources, and this collected data need to be analyzed for the safety surveillance. Spontaneous reporting (SR) adverse event system databases, large clinical projects and health records databases contain data that may be valuable for timely detection of potential risks associated with drugs, devices, and vaccines. All of the data sources include many different AEs and many medical products, so that any approach designed to identify critical signals of potential harm must have adequate specificity to protect against false alarms yet provide acceptable sensitivity for detecting issues that really need further investigation. The algorithms may seek to identify potential drug-event associations without any prior specifications, to identify events associated with a particular product or set of products, or to identify products associated with a particular event or set of events. A whole range of statistical methods have been applied for data mining and signal detection in pharmacovigilance. Primarily there are frequentists as well as Bayesian approaches to SD. This session will provide guidance to various approaches for signal detection. This session will provide recommendations for using data from post marketing spontaneous adverse event reporting databases to provide insight into safety signals and offer guidance regarding appropriate methods like frequentist and Bayesian approaches to use in various situation.
Mervat Alsous
Applied Science Private University, Jordan
Title: Screening for depressive symptoms in patients with diabetic foot using (CES-D) scale: Across-sectional study
Time : 12:40-13:10
Biography:
Abstract:
The aim of this study was to assess levels of depressive symptoms present in patients with diabetic foot. A convenience sampling method was used to recruit 108 patients with diabetic foot. After having completed the Center for Epidemiologic Studies Depression Scale (CES-D), patients' demographic data and medical history were collected using pre-structured forms. Of the entire sample, 38.9% have CES-D score ≥27 which indicates risk for major depression. Logistic regression analysis showed that retinopathy was significantly associated with increased depressive symptoms among diabetic foot patients (odds ratio 3.41 (p=0.017)). Being on a combination of oral hypoglycemic agents and insulin treatment was significantly associated with lower depressive symptoms (odds ratio 3.38 (p=0.022)). Patients with primary education level have the highest odds ratio among all factors associated with risk for major depression (OR, 4.07; p=0.003). The risk for major depression among patients with diabetic foot in Jordan is high compared to general diabetic population. This was associated with low educational level, retinopathy and not taking combination of oral hypoglycaemic agents and insulin. There is a need for routine screening for depression in patients with diabetic foot to help in the prevention, early detection of depression and even referral to a psychiatrist.
Jyoti B Sharma
Tata Memorial Centre, India
Title: Validation of a modified Naranjo scale for causality assessment of adverse events
Time : 14:10-14:40

Biography:
Jyoti B Sharma has completed her Master’s degree in pharmacology and working as research associate in the Department of Clinical Pharmacology, Tata Memorial Centre, Mumbai, India. She is involved in early phase clinical trials (phase 1) as a young researcher and also a co-investigator and presently coordinating the biosimilar trial. She is also involved in the clinical studies like bioequivalence, therapeutic drug monitoring and collecting the adverse drug reaction data in oncology in renal cancer, hepatocellular cancer, lung and prostate cancer. She has one patent in process and two publications.
Abstract:
Background: Correct causality assessment of adverse events (AE) is extremely important. It assumes even greater significance in drug development because of paucity of information available about a drug’s safety profile. The commonly used Naranjo Scale (NS) for causality assessment has several limitations and tends to rule in favour of a positive causal effect even when adverse events are unrelated to the drug. We have therefore attempted to modify the existing NS for better causality assessment.
Methods: We modified the NS by changing the weightage given to certain responses in the existing scale; providing better resolution to certain responses for delineating related from unrelated events and; modifying the slabs for classification of AEs as definite, probable, possible, doubtful and not related. Categories of possible-definite were considered related, and doubtful not related were considered unrelated. This was piloted in 15 cases with 19 AEs that occurred in patients with solid tumours. Modified NS (mNS) was further validated in a set of 65 cases with 104 AEs. Physician opinion/Micromedex was taken as gold standard for the assessment.
Results: In the 19 AEs, six AEs were described by treating physician as unrelated and 13 as related to the drug in question. mNS algorithm had 100% concordance with physician’s opinion whereas the existing NS had only 73.7% concordance. In the validation set of 104 AEs, mNS was 99% concordant with the physician’s opinion while the existing NS was only 70.20% concordant. It was observed that 37/105 AEs were misclassified by existing NS as related when they were actually unrelated.
Conclusion: The existing NS showed a huge bias towards classifying AEs as related to drugs. The modified algorithm gives better sensitivity and specificity for the causality assessment of AEs.
Cristina Scavone
University of Campania Luigi Vanvitelli, Italy
Title: Risk of gastrointestinal complications associated to NSAIDs, low-dose aspirin and their combinations: Results of a pharmacovigilance reporting system
Biography:
Cristina Scavone is a pharmacist attending the last year of PhD in translational medicine. She plays her scientific research as a PhD student at the Department of Experimental Medicine of University of Campania “Luigi Vanvitelli” and at the Pharmacovigilance and Pharmacoepidemiology Center. Her research activities are directed to the topic of pharmacovigilance and pharmaco epidemiology. Since 2014, she is a member of the Italian Society of Pharmacology.
Abstract:
Gastrointestinal (GI) complications are one of the most limiting causes of use of NSAIDs. Beyond others well defined factors, history of peptic ulcer, older age, Helicobacter pylori infection and use of gastrotoxic drugs may affect their GI safety profile. In particular, the risk of GI complications associated to the use of antiplatelet drugs, especially low-dose acetyl salicylic acid (LDA) should deserve much attention. However, only few studies have focused on the effect of combination LDA/NSAIDs on the GI tract compared with the monotherapy and much less studies assessed this effect with multiple NSAIDs use. We aimed to characterize the GI safety profile of NSAIDs and LDA as monotherapy or their combinations in real-life conditions by analyzing spontaneous adverse drug reactions (ADRs) reporting system in a Southern Italy. We used the case/non-case method in the Italian Pharmacovigilance Network (RNF). Cases were reports of GI events in the RNF between January 2007 and December 2011. Non-cases were all other reports during the same period. The association between NSAID and suspected GI ADRs was calculated using the reporting odds ratio (ROR) with 95% confidence intervals as a measure of disproportionality hile adjusting for age, and concomitant use of antineoplastic agents or drugs for cardiovascular diseases. Sub-analyses were performed within the NSAID class. Among the 2816 adverse drug reactions recorded, we identified 374 (13.3%) cases of GI complications. Upper GI complications were the most frequently reported type of events. The highest associations were found for the combined use of NSAIDs and/or LDA, whilst the lowest associations were for their respective monotherapy. Looking at individual NSAIDs the highest association with GI events was observed for ketorolac exposure followed by nimesulide, diclofenac, aspirin, Ketoprofen, and ibuprofen. This study highlights the primary role of the national spontaneous reporting system to bring out potential signals, such as the inappropriate drug use pattern, which however, have to be furtherly studied in-depth with ad hoc population-based studies.
Kety Mirkovic Kos
PharmaKos d.o.o., Croatia
Title: aRMM – Croatian experience on challenges for industry to overcome

Biography:
Kety Mirkovic Kos is a passionate multilingual pharmacovigilance and regulatory expert with comprehensive domestic and international work experience and 60+ completed courses and trainings in the fields of pharmacovigilance, regulatory affairs management, consultancy and quality assurance.
Abstract:
Additional risk minimisation measures pose a challenge to both industry and the regulators. Applicants are obliged to actively monitor safety profile of their medicinal product and to anticipate its important safety risks, based on their own experience or information already available for originator products licensed in the EU. Croatian legislation on pharmacovigilance was last updated around the time of accession to the EU. Any further update is presented through the publicly available news section on HALMED website. Especially news concerning nationally licensed medicinal product are obliging for MAHs for Croatia. Important safety information is usually first communicated to the HCPs via DHCPs and later on in aRMM, following particular country-specific protocols. Request for common aRMM assessments is strongly supported by HALMED, with educational materials on generic name, version and HALMED approval date inserted. Approval costs for the initial aRMM materials and their update is the same, with approval timelines from 6 months to 1.5 years with tendency to decrease. Preparation of common PASS/PAESS studies may be requested by the NCA, irrespective if the licensed medicinal product is marketed in Croatia. What can the industry do to decrease costs and enhance product safety?
Meital Simhi
Ben Gurion University of the Negev, Israel
Title: Mental health treatment preferences among Israeli postpartum mothers
Time : 16:10-16:40

Biography:
Meital Simhi is a PhD student in the faculty of humanities and social sciences at Ben Gurion University. She holds a BA and MA in Social Work from Ben Gurion University. Her PhD study dealt with the relationship between social-demographic characteristics, health beliefs and treatment preferences for PPD among Israeli postpartum mothers. She has presented her thesis results at several conferences. Her PhD research proposal won awards from the ISEF Committee for excellence in education as an outstanding PhD student and from "NA'AMAT", the movement of working women and volunteers, as research that promotes the status of women's health.
Abstract:
The prevalence of Post-Partum Depression (PPD) is 10-20% among new mothers, with rates higher among low income and immigrant populations. Most women do not get treatment for PPD. Antidepressants for women is usually given by a psychiatrist or family physician. The treatment includes selective antidepressants of the Selective Serotonin Reuptake Inhibitor (SSRI) group, which regulates the absorption of serotonin. Among the drugs offered are Lexapro, Lustral, Seroxet, Prozac, and Effectin.The aims of the current study were: 1) describe the preferences for getting mental health treatment for PPD across three dimensions: type of treatment, profession of service provider, and service delivery mode 2) characterize the preferences by elements of health beliefs, health status and cultural group.
Methodology: 1000 women who attended Maternal Child Health Clinics in the Rehovot sub-district for a first medical exam of their infant (9 weeks postpartum) participated in a cross-sectional survey. Data analysis methods: Standard bivariate and multivariate procedures using SPSS.
Results: In this sample, 10.6% of the respondents suffered from PPD as measured by the Edinburgh Postnatal Depression Scale (EPDS), scoring over 9 on EPDS. Depressed mothers were characterized by low income, (p≤0.001 r=0.17,) and medical problems during pregnancy (p≤0.001 r=0.11,). 14.4% of the participants had family psychiatric history, 8.9% of the participants suffered from chronic diseases and 8.2% were taking regular medication. Women who breastfed their infants did not preferred medication treatment (r = -0.14, p≤0.05). Women treated with medication compared to women who did not receive medication preferred to receive professional help in a community treatment centers, (F(3,993)=2.68, p≤..05), while women who were treated in conversations and medical treatment preferred to receive private mental health (F(3,993)=3.91, p≤.05),delivered by mental health professionals(F(3,993)=6.11, p≤.001) and to receive face-to-face treatment (F(3,993)=5.48, p≤..001). Depressed mothers were less likely to prefer treatment in the community treatment center, and they often preferred not to seek treatment at all (r= -0.09, p≤.0.01). In conclusions, women who suffer from PPD represent a high-risk population who can significantly benefit from suitable, accessible treatment. This research clearly indicates the preferences of women in terms of where to receive treatment, from what type of professionals and in which modality.
Mugdha Chopra
APCER Life Sciences, India
Title: Managing challenges and complexities in pharmacovigilance in oncology trials
Time : 15:40-16:10

Biography:
Mugdha Chopra is a dentist by qualification and has over 14 years of experience in the field of dentistry, and pharmacovigilance. She is associate vice president for US PV and clinical safety at APCER and oversees the delivery and operations from India which includes but not limited to case processing aggregate reporting, literature Monitoring and signal detection. Before joining APCER, she was with Ranbaxy as a manager in the Department of Medical Affairs and pharmacovigilance. She comes with an extensive experience in various safety databases like Argus Safety and ARISg for case processing, reporting, and product/license management and has also met the challenges of business continuity planning and data restoration.
Abstract:
Oncology studies are required to be very intensively monitored than studies from many of the other therapeutic areas, because of the nature and severity of the disease and the complexity and potential toxicity of the treatment or the use of newer approaches such as biologics and personalized medicine. In oncology trials unlike the clinical trials in many of the other therapeutic areas, a higher number of subjects are likely to develop serious adverse events and these cases are often complex. The evaluation of drug event relationship and distinguishing from underlying disease and concomitant therapies can be very challenging. Many oncology trials are conducted in high mortality diseases and at times have efficacy endpoints that could also be reportable adverse reactions. The breaking of the blind for such cases as required for expedited reporting to regulatory authorities could compromise the integrity of the clinical trial. In such scenarios, it is advisable to reach agreement with regulatory authorities in advance concerning serious adverse events (SAEs) that would be considered disease related and not subject to systematic unbinding and expedited reporting. Making sure patient compliance with the necessities of a clinical study is quintessential to the success of a study meeting its intended objectives. Therefore, it is imperative to have experienced medical oversight throughout the design, implementation and reporting throughout all phases of a clinical development program. Safety monitors provide tactical drug development guidance as well as review key study documents and safety information and act as the prime medical resource to support the investigators and sites involved in the clinical trials. This session will describe the importance of safety in oncology trials, and how a rigorous and thorough monitoring can lead to the smooth conduct of oncology clinical trials.
- Good Pharmacovigilance Practice | Clinical Trials on Various Disorders | Pharmacy Practices and its Challenges | Case Report in Clinical Trials
Location: Atlantis 2
Session Introduction
Maria Amparo Lopez Ruiz
Cardenal Herrera University, Spain
Title: Self-medication practices among parents who attend to a private pediatric clinic

Biography:
Maria Amparo Lopez Ruiz has completed his PhD from Valencia University and postdoctoral studies from CEU Cardenal Herrera Health Sciences Faculty. She obtained her doctorate in Medicine with the doctoral thesis on “Analysis of the use of medication in the paediatric population that visit accident and emergency department” with summa cum laude. She has achieved the qualification of “University Expert in Neonatology” from the Catholic University in Valencia and “Master’s Degree in Neonatology (from de SENeo -Neonatology Spanish Society-)”. She is Medicine Degree Coordinator in CEU Cardenal Herrera University since 2015. She is the director of the “Master’s degree in Neonatal Intensive care and Neonatal Nursing”. She has attended to International Congresses of Pediatrics as a keynote speaker and she has been part of the Organizing Committee member for the 12th International Conference on Pediatric Pathology and Laboratory Medicine, and she has published in reputed international journals.
Abstract:
Self-medication is a common practice and an important problem of public health between the pediatric patients. The total number of patients who attended to the Accident and Emergencies Department during 1 year (from February 2017 to February 2018) was 2388. A total of 1538 patients attended to the private pediatric clinic taking some medication before the visit. The majority of drugs consumed by these patients (self-medication provided by parents or caregivers of children) were pain-killers and antipyretics (64.8%), followed by drugs for the respiratory system (20.1%) and drugs for the digestive system (11.2%) and antibiotics in a minority form (3.9%). The 1538 self-medicated patients, 143 had some adverse drug reaction due to the medication they were taking; and 35 of these reactions were due to self-medication. The patients from 1 to 4 years were the most self-medicated group (65.1%), followed by the group of 5 to 14 years (22.8%) and those under one year (12.1%). Self-medication among pediatric patients is common; and the most often with pain-killers and antipyretics under dosing leading to ineffectiveness and adverse reactions that could be prevented with proper use of drugs. Regarding the analysis of the dosage of pain-killers and antipyretics: ibuprofen recorded 343 cases of under treatment (54.6%), 28 cases of overtreatment (4.9%) and 251 cases of correct dosing (40.5%). While paracetamol recorded 200 cases of under treatment (35.9%), 32 cases of overtreatment (5.8%) and 331 cases of correct dosing (58.3%). The most frequent adverse drug reactions observed with self-medication were: vomiting (77.3%), skin rashes (12.5%) and diarrhea (10.2%). None of the adverse drug reactions was serious, and could be solved without requiring hospital admission. Although it should be noted that such adverse reactions could have been avoided if they had not made an inappropriate use of the drugs, due in large part to their own lack of knowledge of the indications of the same.
Saed Amarneh
Laniado Hospital Sanz Medical Centre, Israel
Title: The Prevalence and management of polypharmacy in an acute medical depertment of a district general hospital
Time : 12:40-13:10
Biography:
Saed Amarneh has vast experience in hospital pharmacy and is presently director of pharmaceutical services at Laniado Hospital, Netanya, Israel. His primary degree (BSc) was obtained from Jordan's University of Science and Technology (Amman), and his MSc from The Hebrew University (Jerusalem).His interests include research projects, standard setting and lecturing. He is especially interested in improving standards in the hospital pharmacy and introducing clinical pharmacy to all hospital departments. He has a number of publications reflecting his interests
Abstract:
Statement of Problem: Many problems exist with polypharmacy, (the use of four or more medications).= Polypharmacy is associated with an ageing population and multimorbidity. Polypharmacy increases the risk of falls, confusion, functional decline; altered metabolic function; adverse drug interactions; serious side effects, and hospitalisation. Purpose: To determine the prevalence of polypharmacy. To determine the study’s influence on physicians. To determine the percentage of polypharmacy in-patients having a medication review. To document the main reasons for medication changes.
Methodology: Patients’ data was collected over two seven-week periods from one department. e.g. Name, Date of Birth, Medication numbers on admission and discharge, Medication Review Yes/No. Reasons for medication changes were given by each patient’s physician. The department received feedback after the first period. Findings: The prevalence of polypharmacy over both periods was 55% on admission and discharge. The results show a continuous influence on physicians over both periods. The percentage of patients discharged with net additional medications decreased over each period (P1 & P2) (P1 54%->30% , P2 38%->27%); net reductions increased (P1 25%->37%, P2 10%->28%), as well as those having “reduced medications only” (P1 10%->29%, P2 10%->23%). The percentage of patients having a medication review was 99%. 4. The reasons for medication changes were; Symptoms either controlled or not 55% ; New Indication 38% ; Interactions/Side Effects 7%Conclusions and Significance: The prevalence of polypharmacy remained unchanged because patients with reduced medications still continued with polypharmacy. The high prevalence of medication reviews is good practice and was expected. The low incidence of medication changes due to interactions/side effects was unexpected; other studies have shown this to be the major cause. Reasons maybe [a] multiple physicians involved with care; [b] lack of awareness, unwillingness to deprescribe; [c] physicians’ cultural differences.
Safa Alizadeh
University of Medical Sciences, Iran
Title: Adverse fibromyalgia drug events and leading chronicto emergency pain department (June15-16, visits 2016 Philadelphia) at Farabi Eye Hospital: Original study
Biography:
Safa Alizadeh has finished her education in pharmacy. Her first research was on the effect of Probiotics on Rota virus and cell culture. She continues her work on translational ophthalmology research center and passion in researching and studying about ophthalmology adverse drug effects. Currently, her research focused on some eye diseases and ophthalmology drugs such as drops, ointments and gels. This work presents the novel hypothesis about the relation of science and industry to improve health outcome about the patients.
Abstract:
The aim of this study was to evaluate adverse drug events (ADE) resulting in emergency department visits at Farabi eye hospital. Acute ADEs resulting in emergency department visits have a high prevalence however ophthalmic drugs such as eye drops have also a potential for ocular ADEs. The frequency of emergency department visits due to ADEs, the type of ADE, and the suspected drugs were also investigated. In this study all emergency department patients admitted to the emergency department between 8.00 a.m. and 1.00 p.m. during 7 days were included in the study. The patients’ eye disorders and drug history were evaluated by a pharmacist to detect ADEs. The national yellow card was completed for each ADE. Of the 1631 emergency visits, 5 (0.3%, 95% CI: 0.13 to 0.71%) were drug related. Tetracaine eye drops as a local anesthetic accounted for 4 (80%, 95% CI: 38 to 96%) cases. The last patient overused naphazoline drops for one month although it was prescribed by the ophthalmologist for 5 days to treat redness of the eye, resulting in rebound injection and hyperemia. Because all 5 patients abused their ocular drops without a prescription, the ADEs were preventable. Recovery was the outcome of four ADEs after drug discontinuation and appropriate management. Only one of them required penetrating keratoplasty. The most frequent ocular ADE was corneal involvement. Concise monitoring and proper diagnosis of ophthalmic ADEs may reduce ocular complications. As the most prevalent cause of ADE in our study was tetracaine, it seems that the general population should be educated and warned about the hazards of tetracaine drop over consumption drop use without supervision of a physician. The main problem about the local anaesthetic eye drop use occurred with self-administration of the drop without physician supervision; therefore restriction availability of tetracaine drop without prescription is essential.
Safa Alizadeh
University of Medical Sciences, Iran
Title: Adverse fibromyalgia drug events and leading chronicto emergency pain department (June15-16, visits 2016 Philadelphia) at Farabi Eye Hospital: Original study
Biography:
Abstract:
Raymond R Mattingly
Wayne State University School of Medicine, USA
Title: Pharmacovigilance education for medical students
Time : 14:10-14:40

Biography:
Raymond R Mattingly is a Professor and Chair of Pharmacology at Wayne State University, which has the largest single campus medical school in the USA. He was a course director for medical pharmacology and therapeutics for the medical students from 2007-2015. He is a Member of the Academy of Pharmacology Educators of the American Society for Pharmacology and Experimental Therapeutics (ASPET).
Abstract:
In 2006, the Institute of Medicine reported in Preventing Medication Errors that at least 1.5 million preventable adverse drug events occur each year in the USA. A related concern may be whether there is an increase in poor patient outcomes in July, when newly trained medical residents enter practice. Although most such reports are anecdotal, e.g., there are also peer reviewed studies. For example, the highest risk myocardial infarction patients suffer increased mortality in July in hospitals that are categorized as teaching intensive. Most pharmacology education in US medical schools occurs in the early years of the medical school curriculum through introduction to basic principles of pharmacodynamics and pharmacokinetics and then system-specific drug coverage that is often integrated into system-based pathophysiology units. Clinical pharmacology coverage in later years of the US curriculum is rarely required but more often offered as an elective, whereas a recent study shows that compulsory clinical pharmacology education may be more prevalent in the EU. To provide a consistent and universal introduction to the topics of adverse drug events and pharmacovigilance, we instituted a multi-part module in the year-2 of medical student curriculum. We first provide a concise self-study packet of principles and then have faculty facilitation for case-based, small group discussion sessions, with the adverse event cases specifically designed to integrate material across systems. We assess knowledge through multiple-choice questions in the regular course examinations. Student and faculty evaluation of these sessions has been very positive. The pharmacovigilance curriculum has become a key component of our longitudinal curricular theme on patient safety and quality improvement
Furqan Muhammad Iqbal
Bahauddin Zakariya University, Pakistan
Title: Microwave assisted synthesis and evaluation of polyvinyl alcohol-co-2-acrylamide-2- methyl 1 propanesulfonic acid hydrogel for oral delivery of captopril
Biography:
Furqan Muhammad Iqbal, is working as Assistant Professor of Pharmaceutics in Department of Pharmaceutics, Faculty of Pharmacy, Bahauddin Zakariya University, Multan. He started his carrier in product development and quality control in Pharmaceutical Industry (Pharmacia). Up till now, he has more than 10 year experience in teaching Industrial Pharmacy and conducting pharmaceutical research. He has his expertise in Pharmaceutical Technology regarding drug delivery systems, stimuli responsive polymeric drug carriers and their evaluation. Presently, he is working on Research projects on nanotechnology and drug targeting. He has a keen interest and passion in improving the health by minimizing the adverse effects of new as well as already existing therapeutic agents.
Abstract:
We report a highly swellable copolymeric hydrogel prepared by crosslinking poly- vinyl alcohol (PVA) with 2-acrylamido- 2-methyl-1-propanesulfonic acid (AMPS) by microwave radiation using N, N’-methylenebisacrylamide (MBA) as crosslinker and very low quantities of potassium persulfate (KPS) as initiator. The prepared hydrogels were loaded with captopril and subjected to in-vitro and in-vivo evaluation. The swelling studies were performed at pH 2 and pH 7.4 to determine water absorption at lower and higher pH. The hydrogel formulations with higher proportions of AMPS and appropriate dose of radiation demonstrated maximum swelling. The prepared PVA-co-poly (AMPS) hydrogels were evaluated by FT-IR, SEM, XRD, and thermal analysis (DSC and TGA). The crosslinking in components was confirmed by FT-IR, XRD, TGA and DSC analysis. The in-vitro drug release was measured at wavelength of 205 nm using UV spectrophotometer. Drug release observed was higher at pH 2 than at pH 7.4, due to relatively more swelling capacity at lower pH in Aqueous medium. Pharmacokinetic parameters determined by oral administration to rabbits presented increased AUC, AUMC, MRT, t1/2(el), Vss and HVD for hydrogel formulations as compared to control. We can conclude that polyvinyl alcohol and AMPS polymeric network was developed successfully under the influence of microwave radiations as a controlled release drug delivery system for captopril.